How Far Does Cough Travel and what are the implications for disease transmission? TRAVELS.EDU.VN explores the science behind cough droplet dispersal, offering insights into preventive measures and travel safety, ensuring a secure and informed travel experience. Discover key factors influencing droplet travel distance and learn how to protect yourself.
1. The Science of Coughs and Sneezes: A Droplet Generation Overview
Coughs and sneezes are powerful mechanisms for expelling irritants and pathogens from our respiratory system. However, they also play a significant role in the spread of respiratory illnesses. The slogan “Coughs and Sneezes Spread Diseases,” popularized during the 1918-1920 influenza pandemic, underscores this critical point. A single cough can generate approximately 3,000 droplets, while a sneeze can release an estimated 40,000 droplets. Understanding the dynamics of droplet generation and dispersal is crucial for implementing effective preventive measures.
1.1. The Mechanics of a Sneeze
A sneeze is triggered by irritation of the nasal or throat mucous membranes. This irritation leads to a deep inhalation, followed by the depression of the soft palate and palatine uvula, along with the elevation of the back of the tongue. This action partially closes the passage to the mouth. Air then bursts forcefully from the lungs, expelling mucus containing foreign particles or irritants from the nasal cavity.
1.2. The Mechanics of a Cough
Coughing is initiated by the stimulation of sensory nerve fibers, specifically branches of the vagus nerve, located in the ciliated epithelium of the upper airways. This stimulation can be caused by infection, inflammation, or irritation. Afferent impulses from these sensory fibers travel to the medulla, where they are coordinated in the cough center. The efferent pathway involves impulses traveling from the cough center via the vagus, phrenic, and spinal motor nerves to the diaphragm, abdominal wall, and muscles. This complex process results in a forceful expulsion of air designed to clear the airways.
The cough maneuver includes an initial deep inhalation followed by a compression phase. During this phase, the muscles of the chest wall, diaphragm, and abdominal wall contract, and the glottis closes, leading to a rapid increase in intrathoracic pressure. In the subsequent expiratory phase, the glottis suddenly opens, and the high intrathoracic pressure generates an initial high expiratory airflow (up to 12 L/s). This airflow breaks up mucus into smaller droplets, producing the characteristic sound of a cough.
1.3. Droplet Formation Mechanisms
Droplet formation in the respiratory tract occurs through several mechanisms:
- Shear Stress Instability: High expiratory airflow during coughing creates shear stress on the mucus-air interface, dislodging mucus from the airways and breaking it into smaller droplets. This is most prominent in the trachea, where airflow is highest.
- Dynamic Compression: The airways vibrate due to high intrathoracic pressure, squeezing and loosening mucus and promoting the expulsion of foreign material. Vocal cord vibration also contributes to droplet generation.
- Reopening of Collapsed Airways: During normal tidal breathing, droplets can form as collapsed terminal airways reopen at the beginning of inspiration.
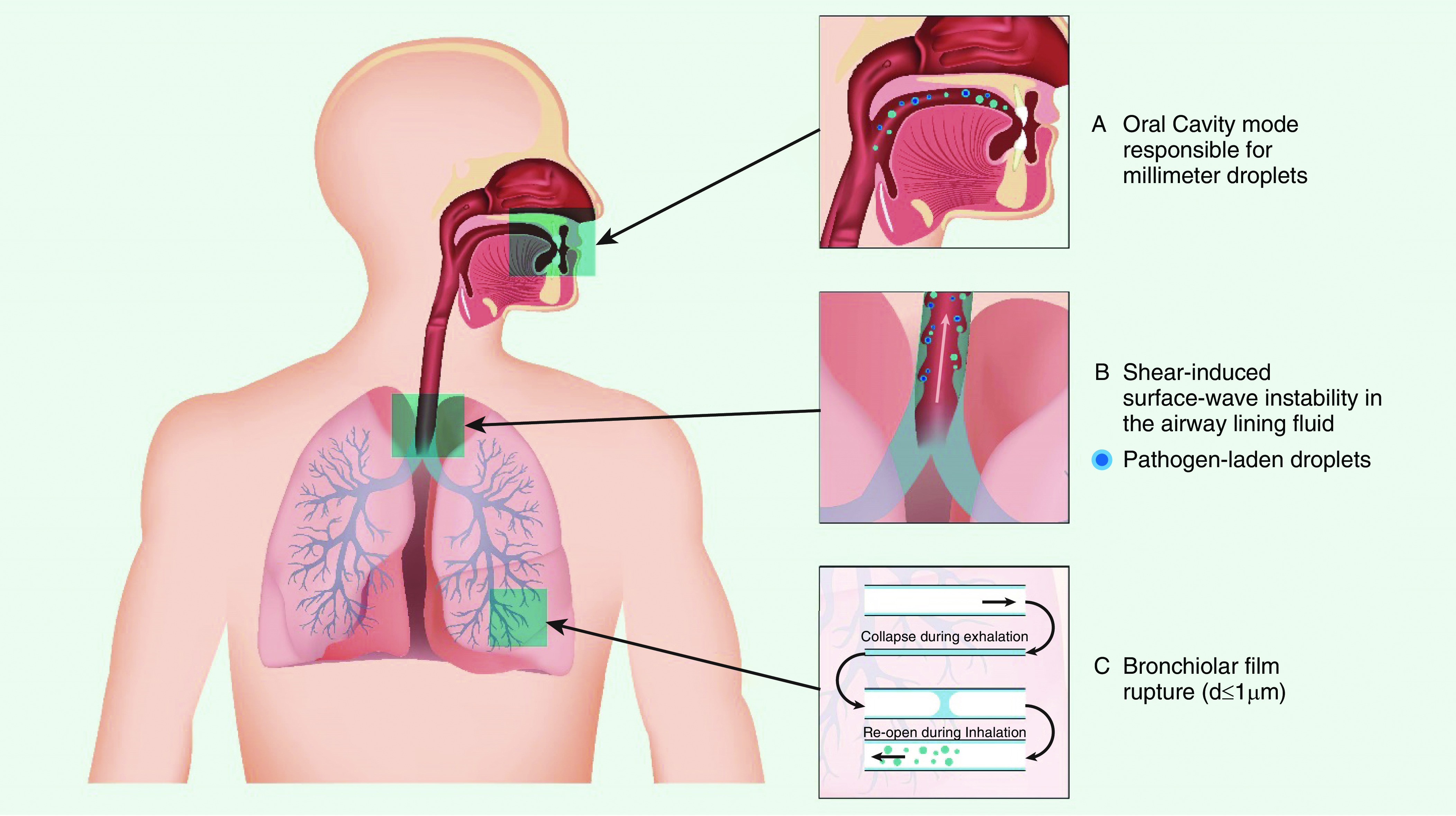 Figure 1 showing the site of origin and mechanisms of droplet generation from the respiratory tract.
Figure 1 showing the site of origin and mechanisms of droplet generation from the respiratory tract.
Figure 1: Illustration of droplet generation from respiratory tract, adapted from Reference 10, showing the site of origin and mechanisms.
2. Factors Influencing Droplet Travel Distance: Understanding the Spread
Several factors influence how far cough droplets travel, impacting the risk of infection transmission. These include droplet size, the force of the cough, environmental conditions, and ventilation.
2.1. Droplet Size and Composition
The size and content of expelled droplets vary depending on their origin. The oral cavity produces larger droplets (around 100 μm) during speech and coughing, while smaller droplets (1 μm) originate in the bronchioles during normal breathing and the larynx during talking and coughing. A study reported the size of coughed droplets to be between 0.62 and 15.9 μm, with an average mode size of 8.35 μm. Viral infections can alter the distribution of particle sizes.
2.2. The Role of Speaking and Asymptomatic Spread
Even loud speaking can produce thousands of fluid droplets per second from the oral cavity, with at least 1,000 droplet nuclei containing virions. These can remain airborne for more than 8 minutes under experimental conditions. Notably, individuals infected with influenza virus exhale aerosol particles containing infectious viral particles more frequently after coughing than after a forceful exhalation. This highlights the potential for “super spreader” events, where an individual infects an unusually large number of people. Other factors include the frequency of respiratory events, viral concentration in exhaled fluid, its volume, and the duration of exposure to an infected individual. Breathing and speaking, which occur more frequently than coughs and sneezes, play a significant role in viral transmission, especially from asymptomatic individuals.
2.3. How Far Can Cough Travel? Understanding the Distance
Larger droplets settle quickly, while smaller airborne droplet nuclei can travel longer distances. Large respiratory droplets containing pathogens like influenza can travel approximately 6 feet when a sick person coughs or sneezes. Cough emissions emerge as a jet with a leading vortex, similar to a puff from a pressurized metered-dose inhaler, penetrating a considerable distance into the surrounding air before dissipating. These emissions contain droplets of various sizes suspended in a turbulent buoyant cloud. Turbulence sweeps around smaller particles, and eddies within the cloud resuspend the particles, causing them to settle more slowly. Some particles can travel more than 8 feet horizontally through the air. Smaller droplets can spray 13–20 feet vertically, potentially entering and traveling through ceiling ventilation systems in some buildings.
2.4. The Impact of Evaporation and Time
Most droplet transmission occurs at close range due to dilution and inactivation of viruses over time and distance. Large droplets between 60 and 100 μm are expected to evaporate completely before traveling 2 meters. High-velocity expulsion, as with coughs and sneezes, can carry these droplets farther. The time it takes particles to fall depends on their size: 100 μm particles take about 10 seconds, 10 μm particles take approximately 17 minutes, and 1- to 3-μm particles can remain suspended almost indefinitely. Infectious droplets carried by airflow from an air conditioner were suggested to have transmitted SARS-CoV-2 among diners at adjacent tables in a restaurant.
3. Routes of Transmission: Airborne vs. Contact
Understanding the different routes of transmission is vital for implementing effective prevention strategies. Respiratory viruses spread through multiple modes, including contact and airborne transmission.
3.1. Contact Transmission
The SARS-CoV-2 virus can last for up to 4 hours on copper surfaces, 24 hours on cardboard, and 2–3 days on less porous surfaces like plastic and stainless steel. Contaminated surfaces can be a source of transmission if individuals touch them and then touch their mouth, nose, or eyes. Indirect transmission through contaminated objects in a shopping mall in China was likely responsible for a cluster of COVID-19 cases.
3.2. Respiratory Droplet Transmission
This is the most common mode of spread for respiratory viruses. Virus-laden droplets, generated by coughing, sneezing, or talking, are propelled directly onto the mucosal surfaces of a host. These droplets are larger and generally fall to the ground after traveling short distances. Transmission can also occur indirectly if a host touches a contaminated surface and then touches their face.
3.3. Airborne Transmission
Airborne transmission occurs when virus-laden fine respiratory droplets remain viable in the environment and are inhaled by a susceptible individual. This can happen directly through inhalation of fine droplets expelled from an infected person or during aerosol-generating procedures on an infected individual. Larger droplets can evaporate, leaving smaller droplet nuclei containing infective microorganisms suspended in the air for extended periods. These can deposit in the lower respiratory tract after inhalation. Larger droplet nuclei that settle can potentially be resuspended after evaporation reduces their size, especially during activities like making a bed or doffing personal protective equipment.
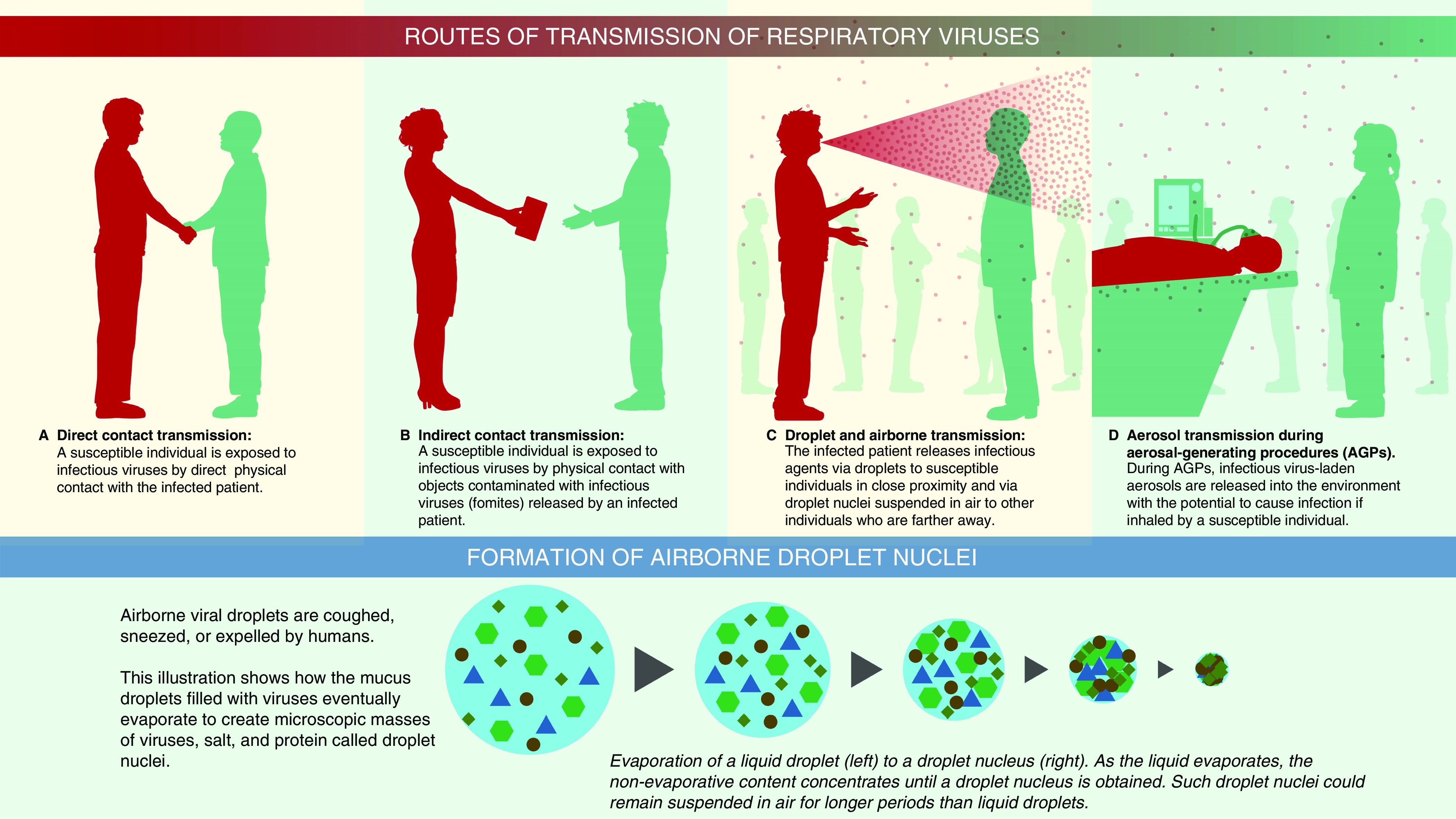 Illustration showing various routes of transmission including contact, respiratory droplet, and airborne.
Illustration showing various routes of transmission including contact, respiratory droplet, and airborne.
Figure 2: Illustration detailing the transmission routes of respiratory viruses, including direct contact, indirect contact, and airborne.
3.4. Environmental Studies and SARS-CoV-2
Viral nucleic acids, and sometimes viable viruses, have been detected in environmental aerosols in healthcare settings. A rising plume of contaminated air, possibly due to suction from an exhaust fan, was thought to cause an outbreak of SARS-CoV-1 in Hong Kong. Preliminary evidence supports airborne transmission of SARS-CoV-2. Viral RNA has been found in the air inside and outside rooms of COVID-19 patients and on ventilation grates. Some studies found the highest virus concentrations in toilet facilities and air outlet fans. Elevated airborne concentrations of SARS-CoV-2 have also been recorded in medical staff areas, suggesting aerosolization during PPE removal. Most environmental sampling studies have detected viral RNA, but few have recovered viable virus, limiting the interpretation of airborne transmission risk. SARS-CoV-2 virus particles have been detected in the air for a median of about 2.7 hours under experimental conditions. The World Health Organization considers SARS-CoV-2 to be transmitted by respiratory droplets and contact, with the virus potentially becoming airborne during aerosol-generating procedures.
4. Particle Deposition in the Respiratory Tract: Where Do They Land?
The size of inhaled particles determines where they deposit in the respiratory tract.
4.1. Filtration Mechanisms
The nose effectively filters inhaled larger particles, while the oropharynx is less effective. Smaller particles have a higher probability of penetrating into the lower respiratory tract. Mouth breathing increases the dose of respirable particles to the lung compared to nose breathing.
4.2. Particle Size Cutoffs
While the optimal particle size for deposition is difficult to define due to changing diameter as droplets travel, some authors propose a diameter of ≤5 μm as a cutoff, though particles of ≤20 μm can desiccate to form droplet nuclei. Most particles >20 μm do not deposit in the lower respiratory tract.
4.3. Factors Influencing Deposition
The mass, diameter, and shape of inhaled particles determine their deposition rate on airway surfaces. Geometric size (d) and density (ρ) are the most important characteristics, influencing the particle’s inertia and transport velocity. Spheres with the same transport velocity exhibit similar aerodynamic behavior and deposition patterns in the lung.
4.4. Mechanisms of Deposition
Particles deposit by impaction, turbulent mixing, sedimentation, and Brownian motion depending on their size. Particles >5 μm in aerodynamic diameter are most likely to deposit by impaction in the oropharynx and be swallowed, while particles <5 μm have the greatest potential for lung deposition. Particles between 4 and 5 μm deposit primarily in the bronchial/conducting airways, while smaller particles remain suspended and penetrate to the peripheral airways and alveoli. In the lung periphery, reduced airflow allows particles to deposit by sedimentation, with gravity causing them to “rain out.” Most particles between 0.1 and 1 μm diffuse by Brownian motion and deposit when they collide with the airway wall. The longer the residence time in the peripheral airways, the greater the deposition from sedimentation and Brownian motion. Inhaled particles that do not deposit are exhaled.
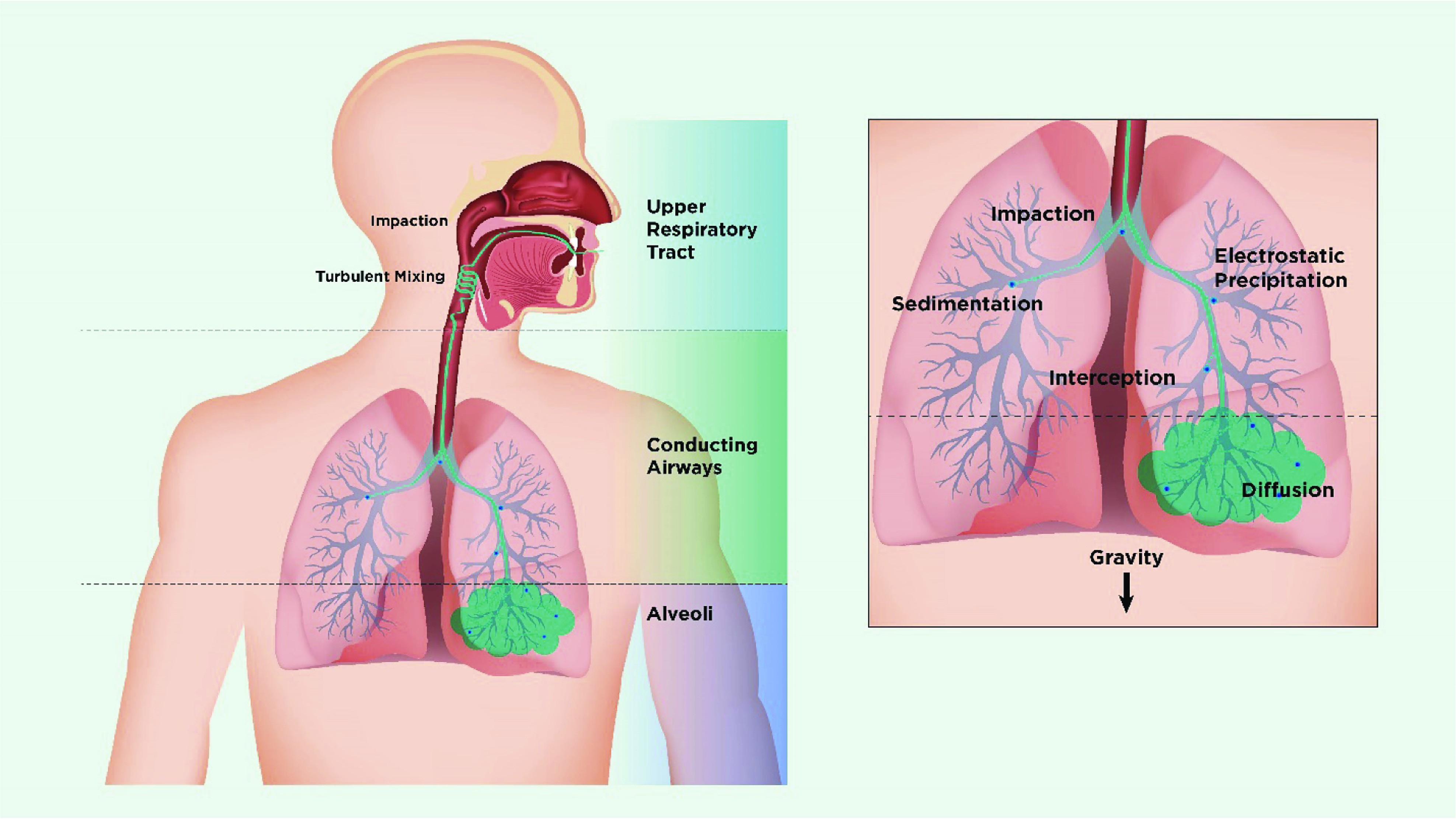 Schematic showing mechanisms of deposition of inhaled particles in the lung.
Schematic showing mechanisms of deposition of inhaled particles in the lung.
Figure 3: Diagram illustrating how inhaled particles deposit in the lungs based on their size and the mechanisms involved.
4.5. Impact of Breathing Patterns and Lung Disease
Inspiratory flow rate influences aerosol deposition, with slow, deep inspirations favoring deeper penetration and fast inspirations targeting the tracheobronchial region. Lung disease affects particle deposition, with higher deposition at obstructed airways and reduced deposition distal to the obstruction.
4.6. Viral Load and Infection Risk
Infection risk depends on the quantity of the pathogen and its site of deposition. Viruses range from 0.02 to 0.3 μm, and bacteria from 0.5 to 10 μm. During tidal breathing, virus particles may be contained in fine particles. A study found that 35% of influenza RNA detected in human coughs was in particles >4 μm, 23% in particles 1–4 μm, and 42% in particles <1 μm. The viral load influences the probability of transmitting infection after inhalation. For COVID-19, the average virus RNA load in oral fluid has been estimated to be 7 × 106 copies/ml. The probability that a 50 μm droplet contains at least one virion is approximately 37%, but this probability is reduced 100-fold in droplets with a diameter of 10 μm. Although very few particles carry pathogens, the number of small particles far exceeds the number of larger-sized droplets.
4.7. Role of Exhaled Breath and Fine Particles
Quantitative PCR has found greater influenza copy numbers in the fine (<5 μm) fraction of exhaled breath compared to the coarse (>5 μm) fraction. This suggests that the infectious dose of influenza via aerosol may be lower than that with large droplets because fine particles are more likely to deposit in the lower respiratory tract. Infected individuals expel fine droplets (count median diameter, 0.57–0.71 μm; geometric SD, 1.54–1.83) with cough, and they produce more particles when they cough compared to when they are healthy. Particles from infected persons contain viable virions.
4.8. Environmental Factors and Humidity
Relative humidity can alter the particle’s aerodynamic diameter, length of time airborne, and viability. A 1.5-μm hygroscopic particle increases to 2.0 μm in diameter on passage through the nose and to 4.0 μm in the saturated air of the nasopharynx and the lung. Microorganisms are hygroscopic, and growth in particle size within airways could increase their retention in the tertiary bronchioles and alveolar ducts. This change is especially significant for viral aerosols because they are highly infectious for the peripheral airways in the lung. Other indoor environmental factors include temperature, ventilation, room size, air exchange frequency, air turbulence, ultraviolet radiation, inorganic and organic contents, exposure duration, the type of virus, and the use of disinfectants.
Table 1: Commonly Used Terminology to Describe Characteristics of Aerosols
| Parameter | Abbreviation | Comments |
|---|---|---|
| Aerodynamic diameter | AD | The AD is the diameter of a fictitious sphere of unit density (1 g cm−3) that has the same gravitational (settling) velocity in the same gas as the actual particle. |
| AD = d (sg)1/2; where sg = ρparticle/ρwater. | ||
| For particles of unit density, the AD is the same as the physical diameter. | ||
| Mass median aerodynamic diameter | MMAD | The MMAD divides the aerosol size distribution in half by mass. It is the diameter at which half the mass of the aerosol particles is contained in particles with larger diameter and the other half in particles with smaller diameter. |
| Geometric SD | GSD | GSD is a measure of dispersion of particle sizes within an aerosol. The GSD is the ratio of the median diameter to the diameter at ±1 SD from the median diameter. In a cumulative distribution plot of the AD and mass of particles, the GSD is calculated as the ratio of the median diameter to the diameter at 15.9% of the probability scale, or the ratio of the diameter at 84.1% on the probability scale to the median diameter. Aerosols with GSD ≥ 1.22 are considered polydisperse. |
5. Aerosol-Generating Procedures (AGPs) and Infection Risk
Certain medical procedures can generate or disperse infectious bioaerosols, potentially increasing the risk of transmission.
5.1. Bioaerosol Generation vs. Dispersion
AGPs like intubation, bronchoscopy, physiotherapy, and suctioning generate potential infectious bioaerosols by provoking cough and are associated with increased infection rates among healthcare workers. Other AGPs such as oxygen therapy, humidified high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), and manual ventilation via mask are less about “generating” bioaerosols and more about “dispersing” them farther away from the patient. Evidence linking AGPs to the spread of viral infections among healthcare providers is limited by the low quality of the studies.
5.2. Aerosol Therapy and Nebulizers
Aerosol therapy significantly increases aerosol concentration in the patient’s vicinity. Aerosols produced by medical aerosol generators do not contain pathogens unless the device is contaminated. Inhalers, including pressurized metered-dose inhalers, dry powder inhalers, or soft mist inhalers, have a low risk of contamination. However, the range of medications available in inhalers is limited, and drugs like antivirals, antibiotics, mucokinetics, and prostacyclins are only available as solutions that require nebulization. The risk of medical aerosols as an AGP is largely attributable to contamination of nebulizers.
5.3. Contamination Sources
Care providers handling medication and the device may contaminate nebulizers, but contamination from the patient and nebulizer design also play important roles. Small-volume jet or ultrasonic nebulizers that are open to and positioned below the gas pathway can be contaminated by the patient’s secretions or exhaled bioaerosols when directly connected to the patient interface. Vibrating mesh nebulizers generate aerosols via mesh plates that separate the sealed medication reservoir from the patient interface. During nebulization, the aerosol derives from the fluid in the nebulizer chamber and does not carry patient-derived viral particles. Residual drug remaining in jet or ultrasonic nebulizers at the end of treatment could act as a breeding environment for bacteria if the nebulizer remains in the circuit between treatments.
5.4. HFNC and NIV Dispersion Effects
In vitro studies using smoke found that HFNC and NIV dispersed exhaled air, as did other oxygen devices. A randomized, controlled study in ICU patients with bacterial pneumonia found that bacterial counts were similar with an oxygen mask and HFNC. However, it is unclear if these findings apply to viral infections. Personal observations of aerosol concentrations near COVID-19 patients suggest that aerosol masses were not significantly different before and after HFNC use and were further reduced when a surgical mask was placed over the patient’s face. Computational fluid dynamic simulations have reached similar conclusions. However, if the connection of nasal cannula is loose during HFNC, a vented mask is used during NIV, or there is a large leak via the mask during NIV or manual ventilation, the leaking port functions as a jet that may spray the exhaled gas with virus in the ambient air, resulting in a longer dispersion distance. Using a tightly fitting nasal cannula and placing a surgical mask over the patient’s face during HFNC helps reduce the dispersion distance. During NIV, vented masks should be avoided, and a filter should be placed between a nonvented NIV mask and exhalation port or between the resuscitator bag and mask.
Figure 4: Illustration comparing aerosol “generating” and “dispersing” procedures, showing that aerosol travel differs with each type.
6. Prevention Strategies: Reducing Airborne Infection
Preventing respiratory infection involves interrupting bioaerosol transmission in three phases: reducing pathogen release at the source, impeding pathogen transportation, and protecting susceptible persons.
To reduce transmission of respiratory tract viruses, the following steps are recommended:
-
Source Control:
- Wear Masks: Proper use of masks, especially in crowded or poorly ventilated areas, can significantly reduce the emission of respiratory droplets.
- Practice Cough Etiquette: Covering the mouth and nose with a tissue or elbow when coughing or sneezing prevents the spread of droplets.
- Stay Home When Sick: Isolating oneself when experiencing symptoms minimizes the risk of transmitting the virus to others.
-
Impeding Transportation:
- Improve Ventilation: Increasing airflow and ventilation rates in indoor environments helps dilute and remove airborne particles.
- Maintain Physical Distance: Keeping a distance of at least 6 feet from others reduces the likelihood of inhaling respiratory droplets.
- Surface Hygiene: Regularly cleaning and disinfecting frequently touched surfaces can minimize the risk of contact transmission.
-
Protecting Susceptible Individuals:
- Vaccination: Getting vaccinated against respiratory viruses, such as influenza and COVID-19, reduces the risk of infection and severe illness.
- Hand Hygiene: Frequent handwashing with soap and water or using hand sanitizer can prevent the spread of viruses.
- Avoid Touching Face: Minimizing contact between hands and the face reduces the risk of virus transmission through mucosal surfaces.
7. Napa Valley Travel with Confidence: TRAVELS.EDU.VN Can Help
Planning a trip to Napa Valley and concerned about health and safety? TRAVELS.EDU.VN offers curated travel experiences designed with your well-being in mind. We understand the importance of feeling secure while exploring the beauty and charm of Napa Valley.
Here’s how TRAVELS.EDU.VN ensures a safe and enjoyable trip:
- Expert Guidance: Our travel experts stay updated on the latest health and safety guidelines to provide you with accurate information and advice.
- Carefully Selected Partners: We work with hotels, wineries, and transportation providers that adhere to strict hygiene protocols.
- Customized Itineraries: We can tailor your itinerary to minimize exposure to crowded areas and prioritize outdoor activities.
- Flexible Booking Options: Enjoy peace of mind with flexible booking and cancellation policies.
- Comprehensive Support: From pre-trip planning to on-the-ground assistance, we are here to support you every step of the way.
Ready to plan your worry-free getaway to Napa Valley?
Contact TRAVELS.EDU.VN today and let us create a customized itinerary that meets your needs and ensures a safe and memorable experience.
Address: 123 Main St, Napa, CA 94559, United States
WhatsApp: +1 (707) 257-5400
Website: TRAVELS.EDU.VN
Don’t let concerns about health and safety keep you from experiencing the magic of Napa Valley. TRAVELS.EDU.VN is your trusted partner for safe and seamless travel.
8. FAQs: Addressing Common Concerns About Cough Droplet Transmission
-
How far can cough droplets travel in still air?
- Cough droplets can travel up to 6 feet in still air, but smaller particles can travel even further, potentially up to 8 feet or more, due to air currents and turbulence.
-
What size droplets pose the greatest risk of infection?
- Both large and small droplets can pose a risk. Larger droplets tend to settle quickly but can cause infection if they land directly on mucosal surfaces. Smaller droplets can remain airborne for longer periods and penetrate deep into the lungs.
-
Does humidity affect the spread of cough droplets?
- Yes, humidity can affect the spread. High humidity can increase the size of droplets, causing them to settle faster, while low humidity can cause droplets to evaporate and remain airborne for longer.
-
Are masks effective in preventing the spread of cough droplets?
- Yes, masks are highly effective in preventing the spread of cough droplets. They act as a barrier, reducing the distance and velocity of expelled droplets.
-
How does ventilation impact the transmission of respiratory viruses?
- Good ventilation helps to dilute and remove airborne particles, reducing the concentration of viruses in the air and lowering the risk of transmission.
-
Can asymptomatic individuals spread respiratory viruses through cough droplets?
- Yes, asymptomatic individuals can spread respiratory viruses through cough droplets, especially during activities like speaking and breathing.
-
What is the role of surface contamination in the spread of respiratory viruses?
- Surface contamination can play a role, as viruses can survive on surfaces for varying periods. Touching a contaminated surface and then touching the face can lead to infection.
-
Are aerosol-generating procedures in healthcare settings a significant risk for virus transmission?
- Aerosol-generating procedures can increase the risk of virus transmission, especially if proper precautions are not taken. Using appropriate protective equipment and ventilation is crucial.
-
How long can respiratory viruses remain viable in the air?
- The viability of respiratory viruses in the air depends on factors such as temperature, humidity, and the presence of UV radiation. Some viruses can remain viable for several hours.
-
What are the key strategies for preventing the spread of respiratory viruses through cough droplets?
- Key strategies include wearing masks, practicing cough etiquette, maintaining physical distance, improving ventilation, and practicing good hand hygiene.
9. Conclusions
Coughs and sneezes generate respiratory droplets of varying sizes that can spread viral infections. These droplets are dispersed into the environment and can be inhaled by susceptible hosts. While larger droplets are often filtered by the nose or deposit in the oropharynx, smaller droplet nuclei can become suspended in room air and inhaled by individuals farther away. These finer particles are carried into the lungs, where their deposition depends on their size and shape. Various procedures and aerosol generators can also produce airborne particles. Preventing respiratory viral infections depends on understanding their propensity to be carried in respiratory droplets or as fine droplet nuclei (airborne transmission). The respiratory transmission of the SARS-CoV-2 virus that causes COVID-19 is primarily through respiratory droplets, though airborne transmission is possible under certain circumstances. Appropriate protective measures are essential to prevent SARS-CoV-2 virus transmission in various settings. travels.edu.vn is committed to providing you with the information and resources you need to travel safely and confidently.