Electromagnetic energy, encompassing light, electromagnetic waves, and radiation, manifests in various forms and plays a crucial role in the universe. From the warmth of the sun to the signals that power our smartphones, understanding how electromagnetic waves propagate is essential. But What Can Electromagnetic Waves Travel Through? This article delves into the fascinating world of electromagnetic waves, exploring their properties and the mediums, or lack thereof, they can traverse.
Electromagnetic vs. Mechanical Waves: A Key Distinction
To understand the nature of electromagnetic wave propagation, it’s helpful to contrast them with mechanical waves. Mechanical waves, like sound waves in air or water waves, require a medium – a substance or material – to travel. These waves are generated by disturbances or vibrations within the medium, whether solid, liquid, gas, or plasma. The molecules within the medium bump into each other, transferring energy like a chain reaction. A classic example illustrates this principle: sound waves cannot travel through the vacuum of space because there is no medium present to facilitate their transmission.
 Water droplet creating ripples in a pool, illustrating mechanical wave propagation.
Water droplet creating ripples in a pool, illustrating mechanical wave propagation.
Classical mechanical waves transfer energy without permanently displacing the medium’s particles. Imagine ripples in a pond; the energy moves through the water, but the water molecules remain largely in place.
The Unique Nature of Electromagnetic Waves
Electromagnetic waves, unlike mechanical waves, possess a unique characteristic: they do not require a medium to propagate. This fundamental difference arises from their very nature.
Electricity, such as static electricity, and magnetism, like that of a refrigerator magnet, are intertwined. A changing magnetic field generates a changing electric field, and vice versa. This dynamic interplay between electric and magnetic fields creates electromagnetic waves.
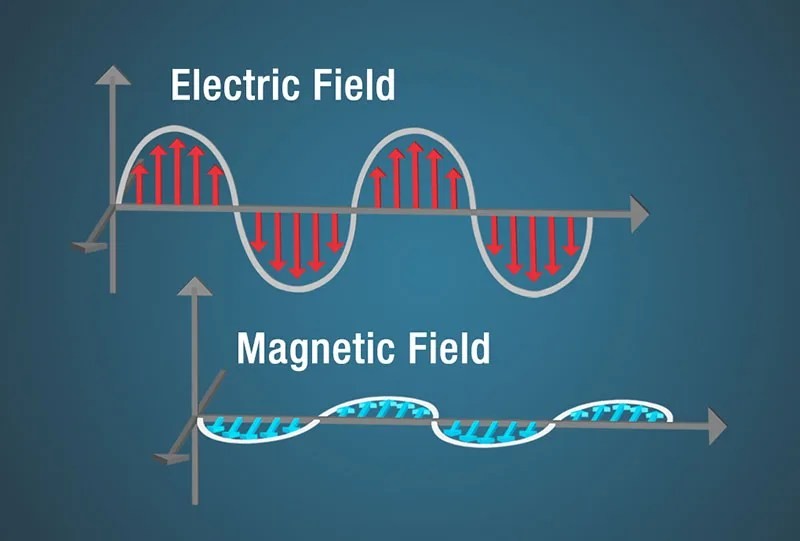 Illustration of electric and magnetic fields oscillating perpendicularly in an electromagnetic wave.
Illustration of electric and magnetic fields oscillating perpendicularly in an electromagnetic wave.
This self-sustaining oscillation allows electromagnetic waves to travel through the vacuum of space, as well as through air and solid materials. This groundbreaking discovery, elucidated by James Clerk Maxwell in the 1860s and 1870s, revolutionized our understanding of light and the universe. Maxwell’s equations elegantly summarized the relationship between electricity and magnetism.
Heinrich Hertz further validated Maxwell’s theories through experiments with radio waves. His work not only confirmed that radio waves travel at the speed of light, thus proving they are a form of light, but also demonstrated how to generate and detect these waves. The unit of frequency, Hertz (Hz), is named in his honor.
Waves and Particles: The Duality of Light
Light exhibits a fascinating duality, behaving as both a wave and a particle. It consists of discrete packets of energy called photons, which possess momentum, travel at the speed of light, and have no mass. Depending on the experimental setup, either the wave-like or particle-like properties of light become more apparent. For example, diffracting light into a spectrum reveals its wave-like nature, while using detectors in digital cameras to capture individual photons demonstrates its particle-like behavior.
Polarization: Alignment of Electromagnetic Fields
Polarization refers to the alignment of the electromagnetic field in a light wave. In vertically polarized light, the electric field oscillates primarily in the vertical direction. This property has practical applications, such as in polarized sunglasses that reduce glare by blocking light with a specific polarization.
Describing Electromagnetic Energy: Frequency, Wavelength, and Energy
Electromagnetic energy can be described using three interrelated properties: frequency, wavelength, and energy. Knowing one allows you to calculate the other two. Radio waves and microwaves are typically characterized by their frequency (measured in Hertz), infrared and visible light by their wavelength (measured in meters), and X-rays and gamma rays by their energy (measured in electron volts). This convention ensures the use of convenient units.
Frequency Explained
Frequency refers to the number of wave crests passing a fixed point per second. One cycle per second is defined as one Hertz (Hz).
Wavelength Explained
Wavelength is the distance between successive crests (or troughs) of a wave. Electromagnetic wavelengths vary dramatically, ranging from fractions of the size of an atom to larger than the Earth’s diameter.
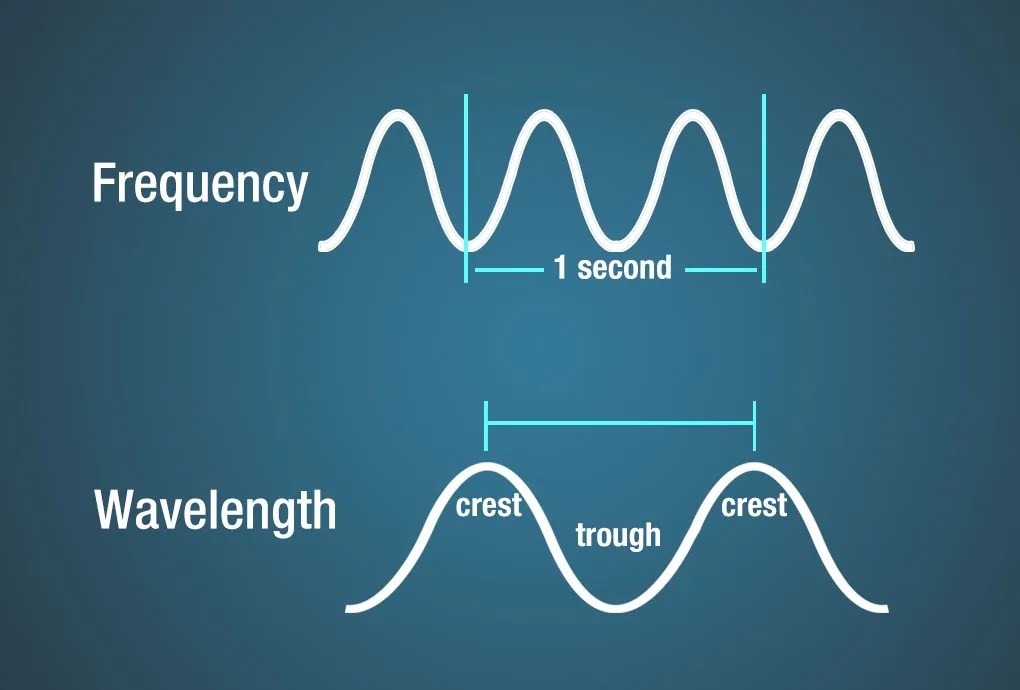 Diagram illustrating the relationship between frequency and wavelength.
Diagram illustrating the relationship between frequency and wavelength.
Energy Explained
Electromagnetic energy is also measured in electron volts (eV), representing the kinetic energy gained by an electron moving through a one-volt potential. Shorter wavelengths correspond to higher energy levels.
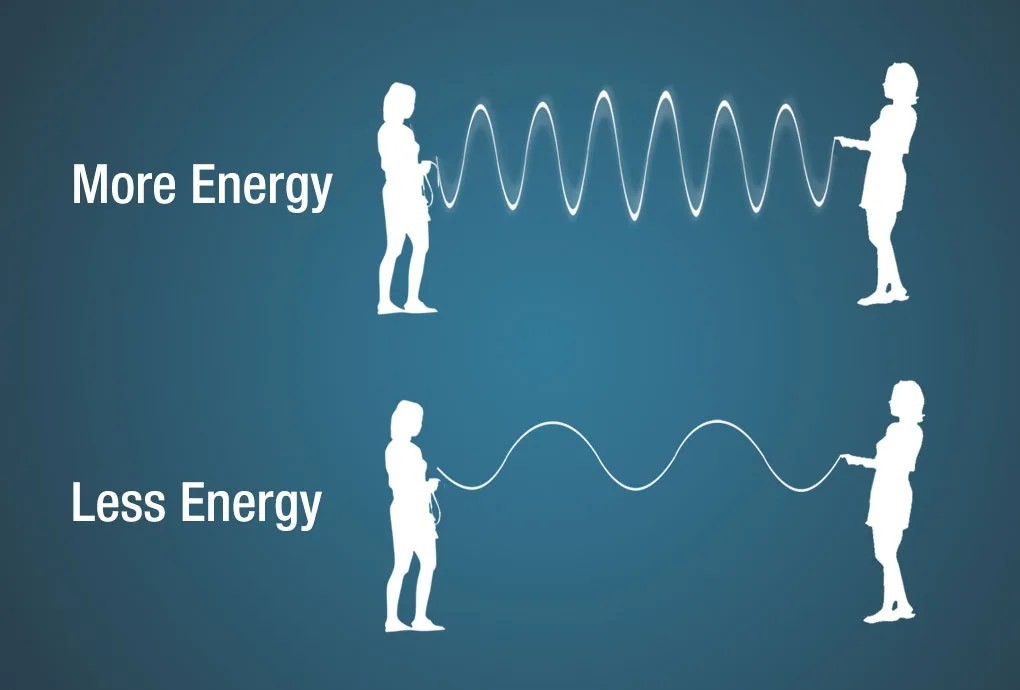 Illustration demonstrating the relationship between energy, wavelength, and frequency using a jump rope analogy.
Illustration demonstrating the relationship between energy, wavelength, and frequency using a jump rope analogy.
Conclusion: The Ubiquitous Nature of Electromagnetic Waves
In summary, electromagnetic waves are unique in their ability to travel through the vacuum of space, as well as through various mediums. Understanding their properties, including frequency, wavelength, energy, and polarization, is fundamental to comprehending a wide range of phenomena, from radio communication to medical imaging. Explore further into the fascinating world of electromagnetic radiation and discover how these waves shape our understanding of the universe. Consider researching specific types of electromagnetic radiation like radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays to see examples of how each of these waves travel through different mediums.